Creamy or Crunchy: Visualizing Food Protein Structures in Wolfram Language

Did you know that protein structures have a major impact on the food we eat?
- Protein structure influences food texture. It can make a food smooth and creamy or crisp and crunchy.
- Protein structure helps determine digestibility. Proteins with looser structures are more readily hydrolyzed into amino acids for easier digestion.
- Protein structure is a factor in whether foods such as peanuts and shellfish cause an allergic reaction.
- Protein structure can make our foods elegant and appetizing.
Explore protein structures with the new Wolfram ProteinVisualization paclet and the BioMoleculePlot3D resource function. Designed for researchers, educators and all structural biology enthusiasts, the paclet offers an immersive experience for viewing the intricate structures of biomolecules, including proteins, nucleic acids and their complexes.
Install and Load the ProteinVisualization Paclet
To download this paclet in your Wolfram Language environment, evaluate the following code:
 Engage with the code in this post by downloading the Wolfram Notebook
Engage with the code in this post by downloading the Wolfram Notebook
To load the code after installation, evaluate this code:
Myoglobin: The First Protein Structure
The science of protein structure began with myoglobin (1MBN). In 1958, John Kendrew and his coworkers reported the first x-ray structure of a protein, that of sperm whale myoglobin. Sixty-five years later, the Protein Data Bank (PDB) now includes more than two hundred thousand structures.
Myoglobin is an example of an oxygen transport protein that contains heme B. Use BioMoleculePlot3D to visualize the 3D ribbon diagram of myoglobin complexed with heme B containing an iron (Fe) atom (in orange) at the center. A ribbon diagram, also known as a Richardson diagram, is one of the most common ways to visualize the 3D structures of proteins. In this representation, the general organization of the proteins is highlighted without the atomic details of the molecule, where secondary structures like α helices or β sheets are shown as coiled ribbons or solid arrows, respectively, and other parts of the backbone are represented as thin tubes. The structure of myoglobin contains only α helices as secondary structures and no β sheets:

The more myoglobin present in a meat, the redder or darker it is. Myoglobin is highest in muscles that are actively used and expend more oxygen. That explains the difference between white and dark meat in poultry. A chicken’s breast muscle is needed only for short bursts of flight and uses less oxygen than the leg muscle, used for daily walking. During cooking, heat denatures the myoglobin protein, changing the color of the meat from red-purple to gray-brown.
Use Wolfram Language to make additional nutrient comparisons between chicken white meat and dark meat, including fat composition:


Milk and Eggs
Alpha-lactalbumin (1F6S)
Alpha-lactalbumin (1F6S) is a major whey protein found in foods made from animal milk, including cheese, yogurt, ice cream and sour cream. Plant-based milks, such as oat milk and almond milk, do not contain whey protein unless it has been added by the manufacturer:

Alpha-lactalbumin’s residue-residue distances are visualized using the ProteinContactMap function of the Wolfram ProteinVisualization paclet. The function creates a customizable contact map for a protein, nucleic acid or protein-nucleic acid complex, displaying residue-residue distances, with options to set the cutoff distance of interactions, display sequence information, define color schemes and more.
Use ProteinContactMap to understand the geometric signatures of different protein structure classes like beta barrel and coiled coil. In its basic usage, the plot is a geometric signature of the shape of the backbone, but you can create a unique fingerprint of a protein by showing the sequence information along with the contact map, as done here. The protein contact map for alpha-lactalbumin illustrates its sixfold symmetry, shown by the diagonal elements of the contact map:

A nutrient comparison in Wolfram Language illustrates the higher proportion of protein relative to calories in Greek yogurt and ricotta cheese. Ice cream has the lowest protein relative to calories, but is worth the treat!

Ovalbumin (1OVA)
The most abundant protein in egg whites, ovalbumin (1OVA) helps form and stabilize foams for whipped cream, meringues and mousses. Some of the ovalbumin unfolds, or denatures, when you whisk egg whites. At the same time, the whisking action adds air and creates bubbles.
Use "RecipeSuggest" from the Wolfram Prompt Repository to create a recipe featuring a foamy meringue:

Plant Proteins
Quinoa (5ZV6)
Quinoa (5ZV6) is considered a complete protein because it contains all nine essential amino acids vital for a healthy diet. Quinoa is an important and historically significant source of protein in many countries. The United Nations declared 2013 as the International Year of Quinoa because South America’s Andean people have maintained their ancestral practice of growing quinoa for present and future generations.
Visualize the ribbon diagram for peptide cQ2 from Chenopodium quinoa, which is a very simple structure containing two antiparallel β sheets:

Dihedral angles are angles between two planes and are an integral part of protein structures, which can be seen here for peptide cQ2 from Chenopodium quinoa using the DihedralAnglePlot function of the Wolfram ProteinVisualization paclet. This function shows the planes involved in the formation of dihedral angles in proteins. DihedralAnglePlot is an interactive way to understand the origin of dihedral angles and the constraints due to steric interactions between side chains of amino acids, as seen here:

After plotting the protein structure, FoodCompassPlot is a quick and easy way to visualize the major nutrient composition of quinoa:

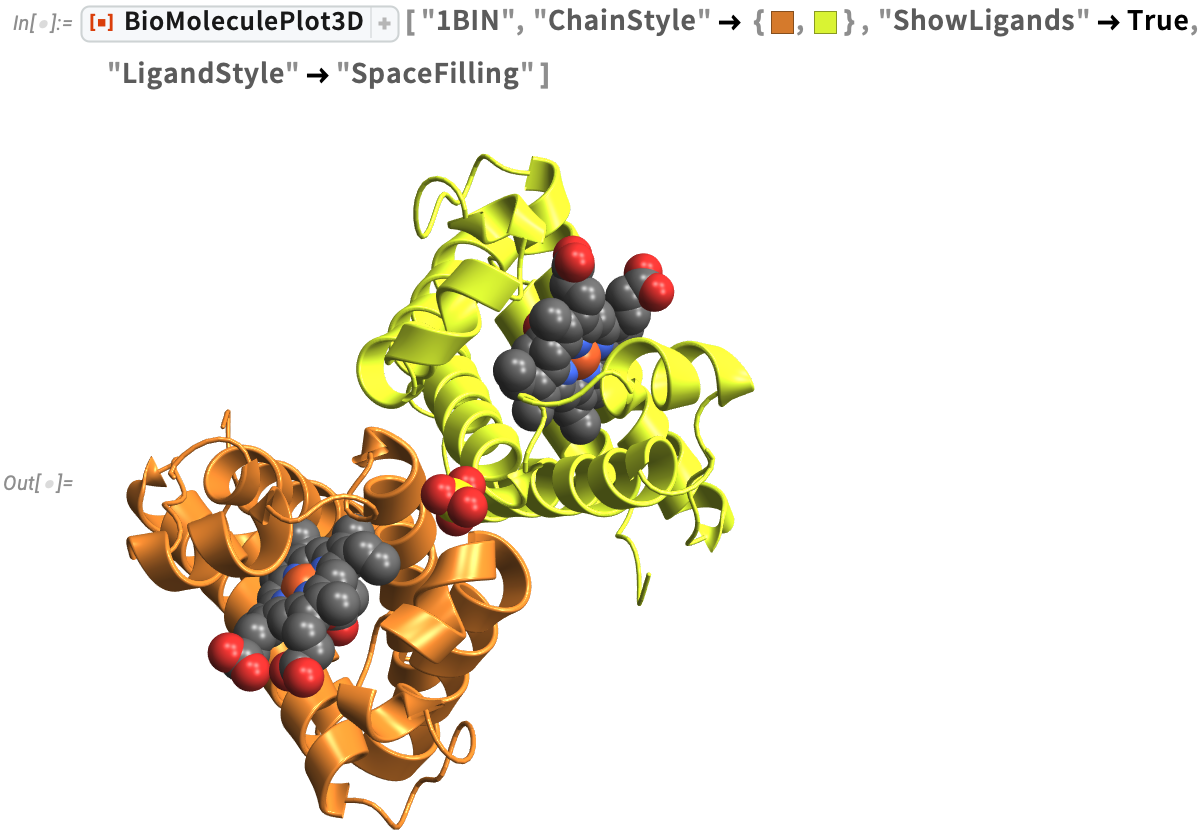
Leghemoglobin A (1BIN)
Found in the roots of soybeans, leghemoglobin A (1BIN) is similar to the myoglobin in meat, which gives meat its flavor and aroma when cooked. Scientists at Impossible Foods have created a type of yeast to make soybean leghemoglobin and give their Impossible™ Burger a meat-like flavor.
Similar to myoglobin, leghemoglobin A is an oxygen transport protein that contains heme B. We can visualize the protein-ligand complex, where the ligand is heme B with an iron atom at the center in orange, using a specified "LigandStyle". Studies of protein-ligand interactions are useful for understanding the mechanisms of biological regulation and can aid in the design of new drugs:
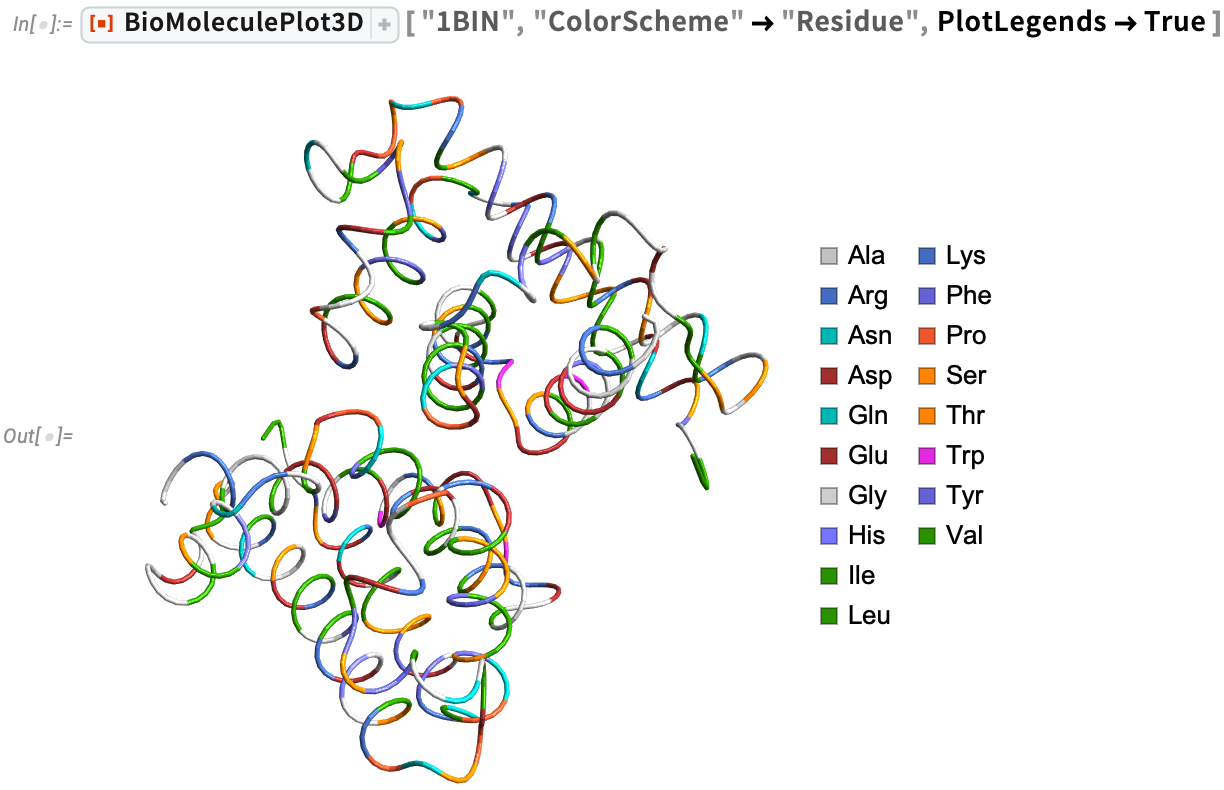
We can also visualize the α-carbon trace of leghemoglobin with color-coded amino acid locations:
Discover the macronutrient composition of more plant-based foods, such as tofu, with NutritionReport:

Food Allergens
Ara h 2 (3OB4)
Ara h 2 (3OB4) is the most potent of the peanut allergens. Peanut allergies affect about 1% of the population and cause the most severe food-related allergic reactions.
Visualize the 3D structure of the protein-ligand complex and the amide planes (highlighting the planar nature of peptide bonds) for the peanut allergen Ara h 2 using BioMoleculePlot3D and AmidePlanePlot, respectively:


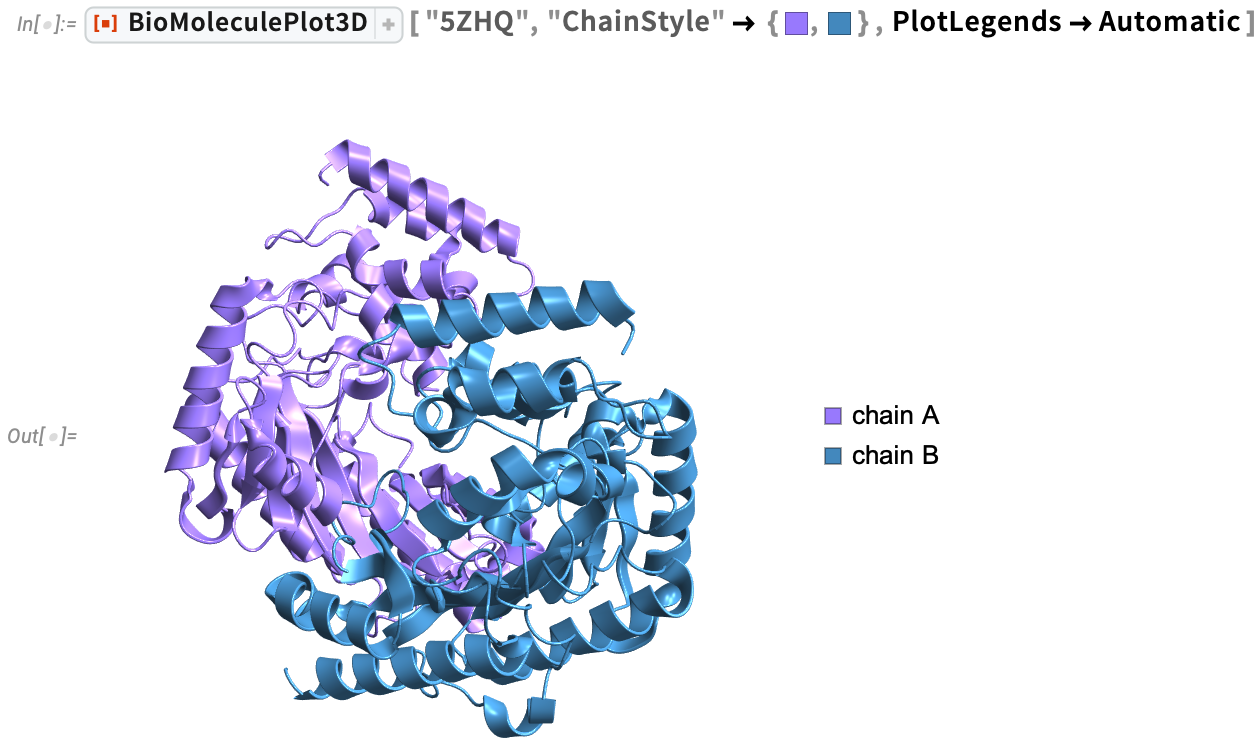
Many people, especially adults, are allergic to shellfish. Tropomyosin is the major allergenic protein across all edible crustacean and mollusk species. Arginine kinases (5ZHQ) is also a major cause of shellfish allergies. Visualize the ribbon diagram of Scylla paramamosain arginine kinase with specific chain colors using BioMoleculePlot3D, then analyze the inter-residue interactions using the ProteinContactGraphPlot function.
In a contact graph plot, the nodes (residues of the biomolecule) are ordered in a circular shape in a clockwise direction. Tooltips provide information about each residue, including chain name, amino or nucleic acid name, and residue number. Information about chains is provided with distinctly colored arrows, where the length of the chain is proportional to the length of the arrow. The directionality of the arrow follows the typical directionality in molecular biology. ProteinContactGraphPlot is a great way to highlight important residues in the protein that are in close proximity to other residues and also visualize which residues are involved in inter-chain interactions:

Enterotoxins
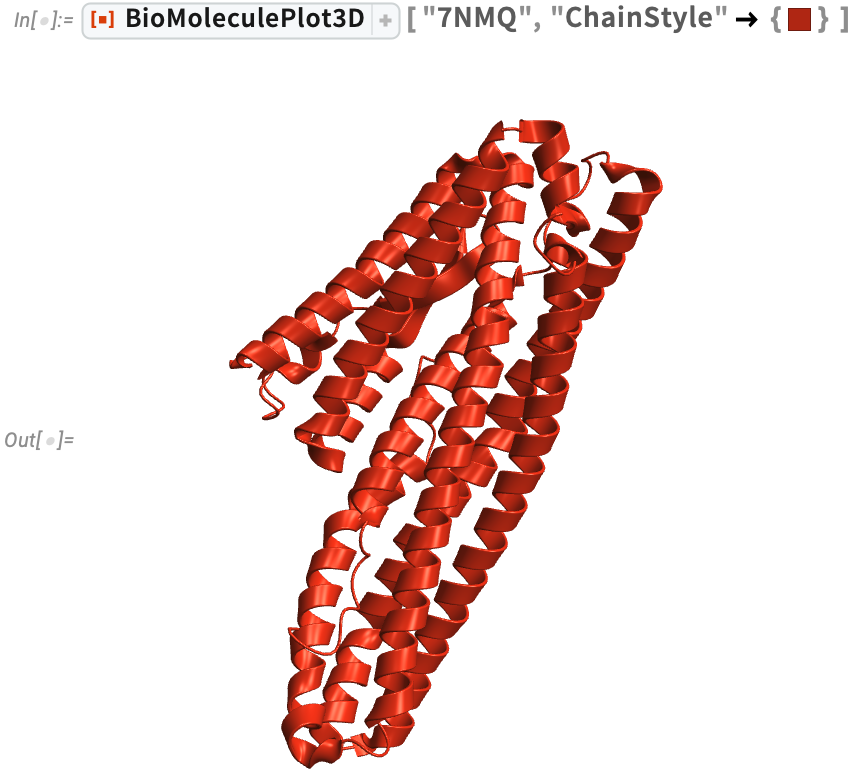
Bacillus cereus (7NMQ)
Bacillus cereus (7NMQ) food poisoning can occur from foods that have been left out at room temperature for extended periods, even if you reheat the food before eating it. The foods most frequently involved are cooked meats and vegetables, boiled or fried rice, custards, soups and raw vegetable sprouts.
Visualize the protein structure and Ramachandran plot for Bacillus cereus HblL1 toxin.This plot shows the distribution of dihedral angles in the protein. Although theoretically the dihedral angle pairs (ϕ, ψ) can take any value from –π to π, the allowed values of dihedral angles in proteins are highly constrained due to steric interactions between amino acid side chains and also inter-residue hydrogen bonding. This can be seen for a given protein using the RamachandranPlot function:

Wolfram Language has two properties that can help you make smart choices about safe food storage:


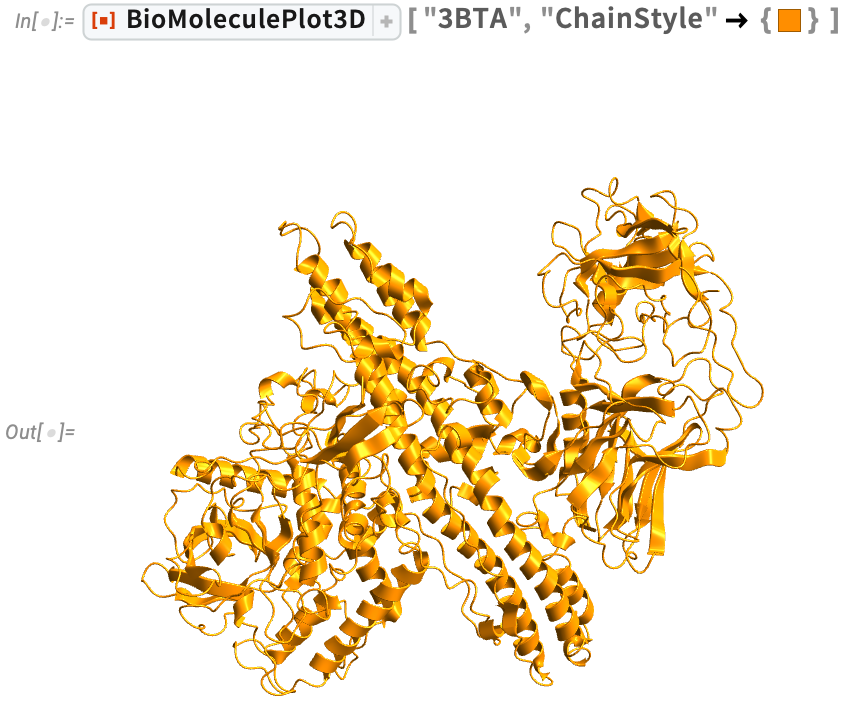
Clostridium botulinum (3BTA)
Clostridium botulinum (3BTA) has multiple roles. It is used beneficially as a treatment for involuntary muscle disorders and to temporarily smooth facial wrinkles. But it can also produce a dangerous neurotoxin that causes the serious, potentially fatal disease of botulism.
Visualize the ribbon diagram and just the amide planes for the crystal structure of botulinum neurotoxin type A. Visualizing solely the amide planes can convey the qualitative organization of the backbone without the details of individual atoms:

Improperly home-canned, preserved and fermented foods are the most common sources of the botulinum toxin. High-risk foods include unrefrigerated homemade salsa, baked potatoes sealed in aluminum foil, chopped garlic in oil and traditionally prepared salted or fermented fish. To reduce the risk of botulism:
- Refrigerate homemade oils that are infused with garlic or herbs, and discard any unused oils after four days.
- Keep hot (above 140° F) any potatoes that have been baked while wrapped in aluminum foil, or refrigerate them with the foil loosened.
- Refrigerate any canned or pickled foods after you open them.
- Do not feed honey to children younger than 12 months. Honey is a primary cause of botulism in infants.
- Follow safe home-canning instructions recommended in the USDA Complete Guide to Home Canning.
Bonus Visualization: Facial Muscles and Botulinum Injections
Want to view the facial muscles most frequently targeted with botulinum injections? According to “Botulinum Toxin Treatment of the Upper Face,” these include the “orbicularis oculi, procerus, corrugator supercilii and frontalis muscles.” Use Interpreter to represent these in Wolfram Language:

Use the AnatomyPlot3D function to view them:

From Food Engineers to Chefs to Home Cooks
Food engineers and scientists must understand the behavior of protein structures in order to develop nutritious and sustainable sources of food proteins. For chefs and home cooks, protein structure affects the gels, emulsifiers and foams they create to elevate their dishes. For all of us, protein structure contributes to the texture, flavor and appearance of the foods we crave most.
| Begin your own culinary adventures with full access to the latest Wolfram Language functionality with a Mathematica or Wolfram|One trial. |

Comments