What’s Your Favorite Element?
Judging elements is like choosing a favorite ice cream. Carbon and hydrogen are like vanilla and chocolate, the basis for so many other flavors, but too commonplace to claim as your preferred element. By using the load-on-demand information packages that are readily available in Mathematica, one can better investigate the popularity of the 118 elements available in ElementData by studying how often they occur in the 34,000 chemicals featured in ChemicalData.

Of all the elements, hydrogen and carbon unsurprisingly occur most frequently, respectively in 94 and 93 percent of the chemicals. As an organic chemist, my focus has traditionally been on carbon-containing molecules, so I cannot help but view the periodic table from a carbon-centered perspective: how will certain elements affect the behavior of molecules to which they are bonded, and how will they interact with other molecules?
Oxygen, nitrogen, and sulfur trail behind at 71, 43, and 12 percent frequency of occurrence. These elements behave as nucleophiles, meaning that they generously share their electrons with other electron-deficient molecules. Electron-sharing allows for bond formation, and alcohols, amines, and thiols (the basic functional groups of carbon molecules containing oxygen, nitrogen, or sulfur) and the generous nature of these elements are often utilized to form esters and amides.
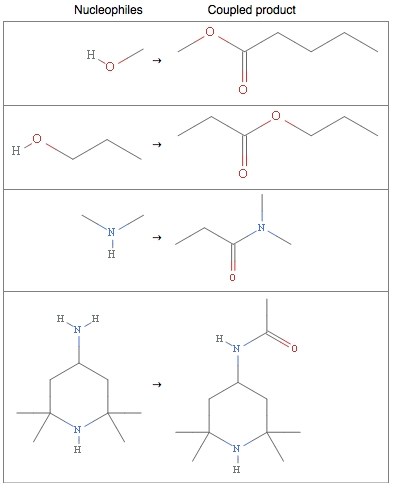
However, as amicable as these elements can be, in combination with each other their behavior changes drastically, often with very reactive results, hence the explosive reactivity of nitro compounds (think TNT), the acidity of sulfuric acid (main component of piranha solution), and the environmentally damaging effects of NOx and SOx pollutants.

Chlorine, fluorine, and bromine occur in 16, 10, and 7.5 percent of the chemicals in ChemicalData. Molecules containing these elements are referred to as “halogenated.” They are common starting materials in organic synthesis because they can easily be knocked off the end of a compound and replaced by another group of atoms (these types of reactions are referred to as substitutions or eliminations). Halogens are good sports about being booted off of a molecule because they already possess seven out of the eight outer-shell electrons that they desire (an octet), and readily acquire their eighth to form chloride, fluoride, and bromide ions.

The majority of the rest of the elements show up less than 1 percent of the time. Some insight into their “social skills” (or lack thereof) can be gained by surveying certain properties such as their ionization energies, electronegativities, and atomic radii.

The more chemically popular elements mentioned earlier have ionization energies that occur in neither the local maxima nor minima. It can be seen that they have some of the highest electronegativities and therefore hold their electrons close, resulting in their relatively small atomic radii, but they are eager to obtain their octets, and are willing to do so by bonding with other atoms to form molecules.
The noble gases (helium, neon, argon, krypton, xenon, and radon) possess full octets, which they guard closely (notice their high ionization energies and small atomic radii), and tend not to interact with other atoms. Due to their antisocial and imperturbable nature they occur in ChemicalData as only their monatomic selves.
The alkali and alkaline earth metals (sodium, potassium, zinc, magnesium, calcium, lithium, and so on) are more popular for use in chemical synthesis than their frequency of occurrence would indicate (<2 percent). These metals contain excess valence electrons, which they loosely possess (large atomic radii) because they are very eager to surrender them. Their low ionization energies make them extremely strong bases—so strong that they will react even with water vapor particles in the air (sodium has been known to ignite violently), and therefore must be generated at the same time as they are used (in situ).

In summary, each element has its own unique personality. Some of them have straightforward behaviors and are outgoing, whereas others can be introverted or mysterious (notice I haven’t mentioned any of the transition elements). To choose a favorite fairly requires getting to know them, and the best way to do this is to take a look at their properties, and the properties of the molecules that contain them.


